
FDA Clears First Apple Watch Medical Device Accessory
The FDA recently cleared the first medical device accessory for the Apple Watch — AliveCor’s KardiaBand. According to AliveCor, the Kardiaband is a mobile, real-time electrocardiogram (EKG) reader that can record an EKG within 30 seconds and display the results on the Apple Watch or iPhone. In conjunction with the KardiaBand, AliveCor is introducing “SmartRhythm, a new feature within the Kardia app for the Apple watch."
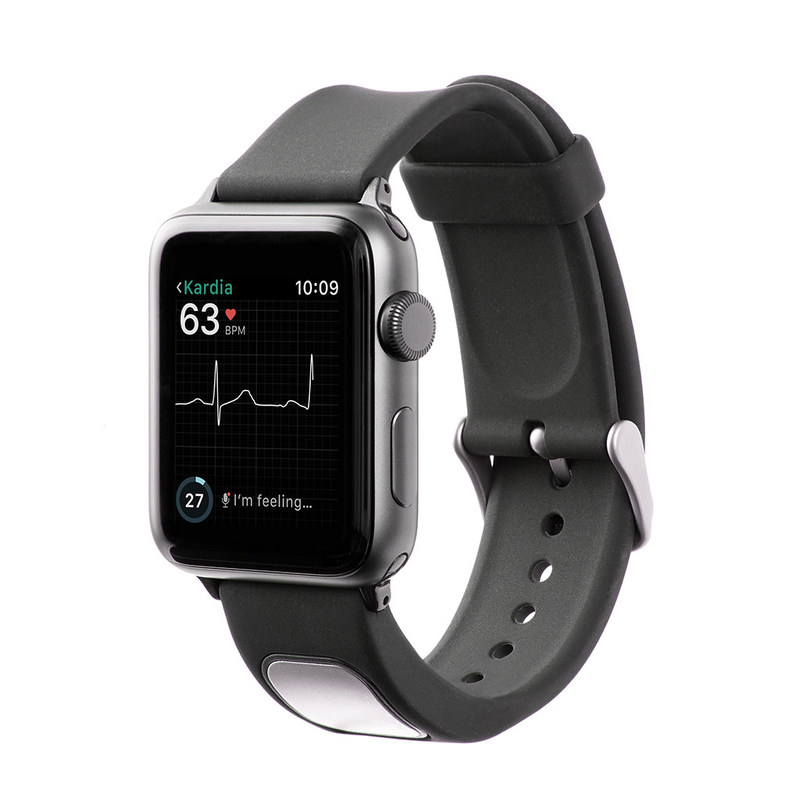
Portable EKG readers for smart phones are not new; several are on the market already. However, none of them can tell the patient when to take an EKG. The KardiaBand solves that problem. The combination of the KardiaBand and SmartRhythm will use the Apple Watch’s sensors “to continuously evaluate the correlation between heart activity and physical activity,” and alert the user to take an EKG using the KardiaBand when the “heart rate and activity are out of sync.” According to Dr. Eric Topol, director of the Scripps Translational Science Institute,
[The KardiaBand] is continuously monitoring your heart rate to let you know if something is potentially off track. That’s the big difference.
AliveCor eplains that the Kardia app will display the results of the EKG on the Apple Watch and alert the patient if atrial fibrillation (Afib) is detected. If necessary, the results of the EKG can be emailed directly to a physician. Dr. Topol believes that this technology will “markedly increase the number of EKGs taken,” which will potentially save lives, because Afib often goes undetected.
The news of KardiaBand’s approval comes within days of Apple’s announcement of the Apple Heart Study in conjunction with Stanford Medicine, which will investigate the detection of Afib using the sensors already embedded in the Apple Watch. However, in a recent interview, AliveCor’s CEO, Vic Gundotra, distinguished the KardiaBand from the heart rate and activity sensors already found on the Apple Watch:
Apple might be able to say ‘oh your heart rate is high’ …but what does that mean? Does that mean you should go to the hospital? And if you go to the hospital what are they going to do?. Any doctor will say ‘ok come in, let’s get an EKG reading.’ . . . It’s not possible to diagnose atrial fibrillation without FDA clearance. That is a big, big play.
AliveCor touts the FDA’s approval of KardiaBand as a reason patient’s can be confident in the results of AliveCor’s mobile real-time EKG technology. In another interview, Mr. Gundotra stated
The average consumer doesn’t know what a normal sinus rhythm looks like or what atrial fibrillation looks like. Yet the FDA has cleared our individual algorithms. The consumer can have confidence that this is FDA-cleared. And frankly, we have the clinical studies to prove it.
The results of a recent study showed a 4-fold increase in Afib detection when using AliveCor’s mobile 30-second EKG technology. That same study showed that when using mobile EKG technology, such as the KardiaBand or AliveCor’s other device, fewer cases of Afib went undiagnosed.
The KardiaBand is available on AliveCor’s website for $199.
