
Tesaro Receives Early FDA Approval for Ovarian Cancer Drug Zejula
On March 27, 2017, the U.S. Food and Drug Administration approved the use of the poly ADP-ribose polymerase (PARP) inhibitor, Zejula (niraparib), for the maintenance treatment of recurrent ovarian, fallopian tube, or primary peritoneal cancer following complete or partial response to platinum-based chemotherapy. The FDA granted the Zejula application after it was accepted for review under Fast Track, Priority Review, Breakthrough Therapy, and Orphan Drug designations. These designations indicate that Zejula is a drug for treating serious conditions, fills an unmet medical need, is a significant improvement in safety or efficacy of the treatment of ovarian cancer, and demonstrates substantial improvement in treatment of ovarian cancer.
Ovarian cancer is the fifth-most frequent cause of cancer death among women, with approximately 22,000 women diagnosed, and 14,000 deaths each year in the United States. Ovarian cancer usually has a relatively poor prognosis, with unsatisfactory treatment outcomes. Approximately 85% of patients experience recurrence within two years. Ovarian cancer is a result of dividing cells in the ovary that acquire gene mutations, causing cancerous growth. The commonest gene mutations in ovarian cancer occur in NF1, BRCA1, BRCA2, and CDK12. Patients with BRCA mutations generally undergo platinum chemotherapy, following by a watch and wait treatment approach.
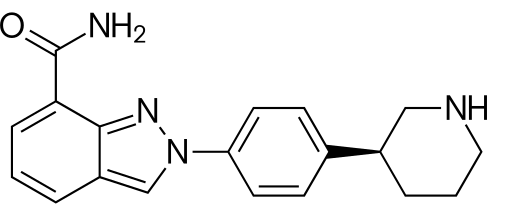
Zejula (2-[4-[(3S)-3-Piperidyl]phenyl]indazole-7-carboxamide; chemical formula shown below) is an orally available PARP inhibitor that blocks PARP enzymes, PARP-1 and PARP-2, which play a role in DNA repair in the BRCA protein pathway.
Zejula demonstrated a clinically meaningful increase in progression-free survival (PFS) in women with recurrent ovarian cancer, regardless of BRCA mutation or biomarker status.
According to a Tesaro press release, “Zejula reduced the risk of disease progression or death by 74% in patients with germline BRCA mutations (HR 0.26) and by 55% in patients without germline BRCA mutation (HR 0.45).” Because Zejula reduces the risk of disease progression regardless of BRCA mutation, patient selection with a biomarker test is not required. In addition, the maintenance treatment is effective in patients who have had either complete or partial response to platinum-based chemotherapy.
Tesaro Inc anticipates launching Zejula in the U.S. in late April 2017.

