On August 2, 2017, the U.S. Food and Drug Administration approved the use of Imbruvica® (ibrutinib) for the treatment of adult patients with chronic graft versus host disease (cGVHD) to Pharmacyclics LLC. Imbruvica® is the first FDA-approved therapy for the treatment of cGVHD.[1]
cGVHD is a condition that may occur in patients who have received a stem cell transplant to treat certain blood or bone marrow cancers, when cells from the transplanted stem cells attack healthy cells in the patient. Previously, patients with cGVHD who did not respond to other forms of therapy, such as corticosteroids to suppress their immune system, did not have a treatment option specifically for the condition. With this approval the FDA has given a second-line nod, meaning patients who do not respond to typical treatments for cGVHD may receive Imbruvica®. This condition is estimated to occur in 30% to 70% of all patients who receive the stem cell transplant treatment.
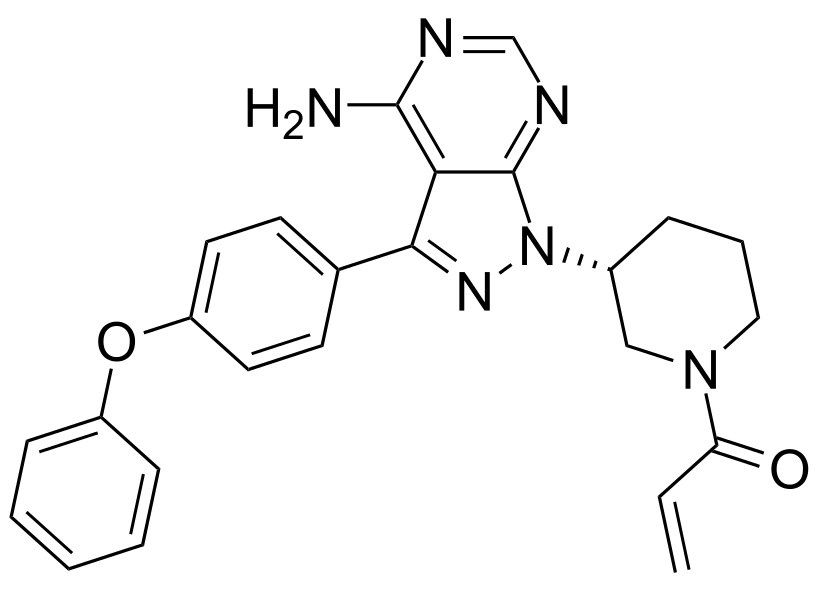
Imbruvica®[2] (1-[(3R)-3-[4-Amino-3-(4-phenoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidin-1-yl]piperidin-1-yl]prop-2-en-1-one) is a kinase inhibitor. The FDA granted Pharmacyclics’s application for Imbruvica® Priority Review and Breakthrough Therapy designations, and well as an Orphan Drug designation for use in treating cGVHD. Prior to approval for treating cGVHD, Imbruvica® was approved for treatment in the blood cancer arena to treat chronic lymphocytic leukemia, Waldenström’s macroglobulinemia and marginal zone lymphoma, and received accelerated approval status for mantle cell lymphoma.
Imbruvica® was developed by Pharmacyclics alongside Johnson & Johnson, with an investment from Abbvie.[3]

