The FDA has granted approval for Novocure‘s wearable medical device, Optune Lua, designed to treat advanced non-small cell lung cancer (NSCLC). The device utilizes portable transducer arrays placed on the skin to deliver alternating electrical fields, termed Tumor Treating Fields (TTFs), thereby disrupting malignant cell division while leaving healthy cells largely unaffected.

Optune Luna was first approved in 2019 under the Humanitarian Device Exemption pathway in combination with chemotherapy to treat malignant pleural mesothelioma, a type of lung cancer that develops in the lining outside of the lungs and is typically attributed to asbestos exposure.
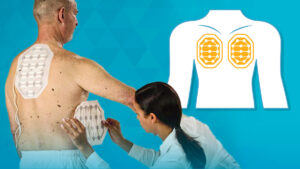
The FDA’s recent approval was granted based on findings from the “Lunar” Phase 3 clinical trial. The study involved patients whose cancer had progressed following first-line platinum-based chemotherapy. The participants were randomly assigned to either receive TTF treatment with the Optune Luna device along with standard therapies—such as chemotherapy or PD-1 inhibitors like Merck & Co.’s Keytruda—or to receive standard therapies alone. Participants using the device alongside standard therapies experienced a 26% lower risk of death during a median follow-up period of approximately 10 months.

Novocure’s TTField technology has also been used as a treatment for a rare type of brain cancer, glioblastoma multiforme (GBM). In 2011, the FDA approved Novacure’s Optune Gio device for treating the recurrence of GBM after treatment with chemotherapy. The new FDA approval of Optune Lua opens up significant revenue potential for Novocure, by allowing it to enter the market for treating NSCLC, a more prevalent cancer type than GBM.
Novocure is exploring additional clinical opportunities for TTFs in other cancers, including brain metastases, gastric cancer, liver cancer, pancreatic cancer and ovarian cancer. While there are inherent clinical and regulatory risks, the positive track record of TTFs in trials may support future expansions in cancer treatment options.
