In addition to its significant efforts to fast track the approval of COVID-19 related devices, the FDA’s Center for Devices and Radiological Health (CDRH) has also maintained its high priority focus on supporting innovation of new medical devices in the United States.
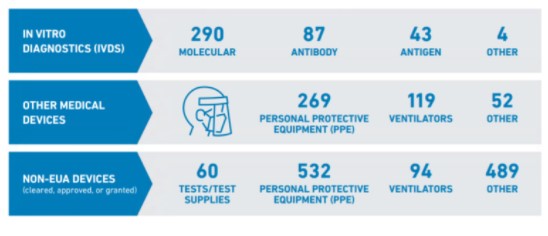
According to CDRH Director Jeff Shuren, M.D., J.D., the FDA has now granted emergency use authorization (EUA) or full marketing authorization to over 2,000 medical devices intended to prevent, diagnose, or treat COVID-19 and more than half of CDRH’s workforce has been directly involved in the COVID-19 response. CDRH moved quickly with respect to COVID-19 treatment devices to ensure the availability of the wide range of related products shown below, including diagnostic tests, personal protective equipment (PPE), ventilators, and other critical devices and supplies for health care providers and patients.
As one example, CDRH authorized 15 additional over-the-counter (OTC) COVID-19 tests for at-home use during 2021. According to CDRH Director Shuren, these were authorized in record times—in some cases in less than a week—highlighting CDRH’s devotion to providing a rapid response to the COVID-19 pandemic.
Importantly, CDRH’s pandemic-related efforts have not brought its other authorizations to a grinding halt. Instead, CDRH also managed to clear or approve 13 devices with breakthrough designation and granted market authorization for 103 novel devices during 2021. “One way that we are measuring the success of our efforts is through tracking the number of innovative medical technologies being brought to the U.S. first so that patients have access to the safest and most innovative devices available,” says Shuren. “A decade ago, the U.S. was too often behind in this regard. But we are gratified to see our efforts have resulted in 103 novel devices receiving marketing authorization in 2021, despite the unprecedented demands of our pandemic response,” continues Shuren. “Spurring innovation in developing safer, more effective devices is key to improving patient care and quality of life.”
Giving marketing authorization to 103 novel devices is touted by the Annual Report as “an incredible achievement” in light of the increased demand on CDRH staff resulting from the COVID-19 pandemic. “This highlights the commitment and dedication of CDRH staff to strengthen public health by bringing innovative devices to patients,” comments CDRH.
In the past ten years, CDRH has issued four times as many approvals, authorizations, and clearances of novel technologies as a result of the innovative policies and approaches developed and implemented by CDRH. Novel technologies include those brought to market through the Premarket Approval, Humanitarian Device Exemption, and De Novo pathways, as well as a subset of those brought to market through Emergency Use Authorization (EUA), or the Breakthrough Devices Program.
The Breakthrough Devices Program is another important program to emerging companies that provides device manufacturers with an opportunity to engage directly with CDRH’s experts through several different program options to address topics as they arise during device development, evaluation, and premarket review. Some of the benefits of this program are expedited feedback to manufacturers and prioritized submission review. By the numbers, in 2021, CDRH granted breakthrough designation to 213 devices, which is roughly one-third of all designations since the program’s inception in 2015, and granted marketing authorization to 13 breakthrough devices (including 3 Premarket Approvals, 3 510(k)s, and 7 De Novos).
Notably, of these devices, CDRH approved the first in the world non-surgical heart valve to treat pediatric and adult patients with a native or surgically repaired right ventricular outflow tract (RVOT), the part of the heart that carries blood from the right ventricle to the lungs. According to the Annual Report, this Harmony Transcatheter Pulmonary Valve (TPV) System is intended to improve blood flow to the lungs in patients with severe pulmonary valve regurgitation without open-heart surgery, which is the current standard of care. Other examples of approved novel technologies can be found in CDRH’s Annual Report.
