Every year, the FDA’s Center for Drug Evaluation and Research (CDER) approves new medications. Some medications are variations of existing products, such as generic formulations or new dosage forms of previously-approved products. Other medications are novel drugs, having chemical structures that have never previously been approved for human use.
Novel Drug List
The CDER publishes a summary of the approved novel drugs on the FDA website. The FDA recently released the summary of the novel drugs approved in 2016 (click here for the full summary). The summary includes 22 novel drugs, approved either as new molecular entities (NMEs) under New Drug Applications (NDAs) or as new therapeutic biologics under Biologics License Applications (BLAs). The table below lists the 22 novel drugs approved in 2016 and related approval information.
|
No. |
Drug |
Active Ingredient |
Approval Date |
FDA-approved use |
Pharmaceutical Company |
Special Designation |
|
1. |
elbasvir and grazoprevir |
1/28/2016 |
To treat patients with chronic hepatitis C virus (HCV) genotypes 1 and 4 infections in adult patients. |
Merck & Co. Inc. |
|
|
|
2. |
brivaracetam |
2/18/2016 |
To treat partial onset seizures in patients age 16 years and older with epilepsy. |
UCB, Inc. |
|
|
|
3. |
Obiltoxaximab |
3/18/2016 |
To treat inhalational anthrax in combination with appropriate antibacterial drugs. |
Elusys Therapeutics, Inc.; Developed |
Drug for rare disease |
|
|
4. |
ixekizumab |
3/22/2016 |
To treat adults with moderate-to-severe plaque psoriasis. |
Eli Lilly and Company |
|
|
|
5. |
reslizumab |
3/23/2016 |
To treat severe asthma |
Teva Respiratory, LLC. |
|
|
|
6. |
defibrotide sodium |
3/30/3016 |
To treat adults and children who develop hepatic veno-occlusive disease with additional kidney or lung abnormalities after they receive a stem cell transplant from blood or bone marrow called hematopoietic stem cell transplantation |
Jazz Pharmaceuticals. |
First in class; Drug for rare disease |
|
|
7. |
Venetoclax |
4/11/2016 |
For chronic lymphocytic leukemia in patients with a specific chromosomal abnormality |
AbbVie and Genentech USA Inc. |
First in class; Drug for rare disease |
|
|
8. |
pimavanserin |
4/29/2016 |
To treat hallucinations and delusions associated with psychosis experienced by some people with Parkinson’s disease |
Acadia Pharmaceuticals. |
|
|
|
9. |
atezolizumab |
5/18/2016 |
To treat urothelial carcinoma, the most common type of bladder cancer |
Genentech USA Inc. |
|
|
|
10. |
Daclizumab |
5/27/2016 |
To treat multiple sclerosis |
Biogen, Inc. |
First in class |
|
|
11. |
obeticholic acid |
5/27/2016 |
To treat rare, chronic liver disease |
Intercept Pharmaceuticals, Inc. |
First in class; Drug for rare disease
|
|
|
12. |
fluciclovine F 18 |
5/27/2016 |
A new diagnostic imaging agent to detect recurrent prostate cancer |
Blue Earth Diagnostics, Ltd., |
|
|
|
13. |
gallium Ga 68 dotatate |
6/1/2016 |
A diagnostic imaging agent to detect rare neuroendocrine tumors |
Advanced Accelerator Applications USA, Inc. |
Drug for rare disease |
|
|
14. |
sofosbuvir and velpatasvir |
6/28/2016 |
To treat all six major forms of hepatitis C virus |
Gilead Sciences, Inc |
|
|
|
15. |
lifitegrast ophthalmic solution |
7/11/2016 |
To treat the signs and symptoms of dry eye disease |
Shire US Inc.
|
First in class |
|
|
16. |
lixisenatide |
7/27/2016 |
To improve glycemic control (blood sugar levels) |
Sanofi-Aventis U.S. LLC |
|
|
|
17. |
Eteplirsen |
9/19/2016 |
To treat patients with Duchenne muscular dystrophy |
Sarepta Therapeutics. |
First in class; drug for rare disease |
|
|
18. |
Olaratumab |
10/19/2016 |
To treat adults with certain types of soft tissue sarcoma |
Eli Lilly and Company |
Drug for rare disease |
|
|
19. |
Bezlotoxumab |
10/21/2016 |
To reduce the recurrence of Clostridium difficile infection in patients aged 18 years or older |
Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc. |
First in class |
|
|
20. |
crisaborole |
12/14/2016 |
To treat mild to moderate eczema (atopic dermatitis) in patients two years of age and older |
Anacor Pharmaceuticals, Inc. |
|
|
|
21. |
rucaparib |
12/19/2016 |
To treat women with a certain type of ovarian cancer |
Clovis Oncology, Inc. |
Drug for rare disease |
|
|
22. |
Nusinersen |
12/23/2016 |
To treat children and adults with spinal muscular atrophy (SMA) |
Marketed by Biogen; Developed by Ionis Pharmaceuticals |
First in class; Drug for rare disease |
Lower Than Average Approval Number
The number of approved novel drugs in 2016 is less than half of the 45 novel drugs approved in 2015. The chart below illustrates the novel drug filings and approvals in each year of the past decade. The vertical bars in the graph indicate the number of novel drugs approved, and the points connected by lines indicate the number of new applications filed. From 2007 through 2015, CDER received an average of about 36 applications for novel drugs per year, while the average approval number remained about 29 drugs per year. While the approval numbers were down, CDER estimated 41 filings for 2016, higher than the average in recent years.
John Jenkins, director of FDA’s Office of New Drugs, who retired from the FDA on January 7, 2017, provided several explanations for the lower number of novel drugs approved in 2016 on the FDA’s blog. One reason he offered was that CDER approved five novel drugs in 2015 that had originally been planned for approval in 2016. Another reason he mentioned was a higher number of Complete Responses (CR) in 2016. Issuance of a CR provides advice on what the sponsor needs to do for FDA to support resubmission of the application. CDER issued 14 CR letters for novel drugs in 2016, higher than in recent years.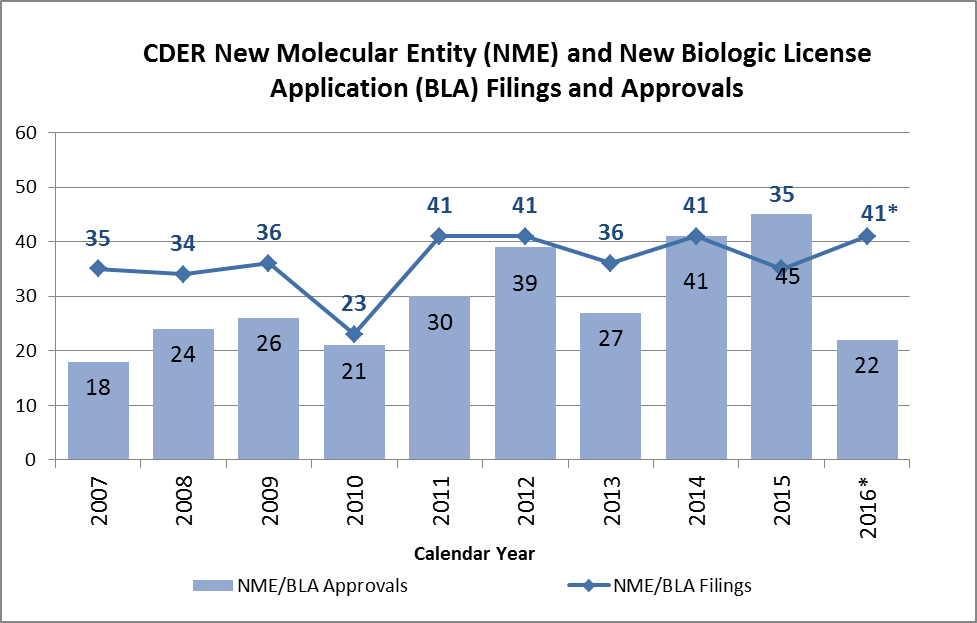
Approval Number Break Down
Despite the lower number of approvals, The FDA’s Novel Drug Summary for 2016 notes that the approved drugs have the potential to have a big impact in several therapeutic areas. Eight of the drugs approved last year were identified as “first in class” treatments, and nine (41%) of the novel drugs were approved as orphan drugs. An orphan drug is one that treats rare diseases that affect fewer than 200,000 Americans. A majority of the novel drugs approved in 2016 benefited from the FDA’s programs to expedite its review process: eight of the twenty-two drugs had a fast track status for drugs having the potential to address unmet needs; seven were considered breakthrough therapies with preliminary clinical evidence demonstrating substantial improvement over other available therapies; fifteen received a priority review designation for review within six months instead of the standard 10 months; and six benefited from the accelerated approval for early approval for a serious or life-threatening illness with benefit over existing therapies. The FDA’s report also noted that 19 of the 22 novel drugs were approved in the United States before receiving approval in any other country.
