On June 25th, 2018, the U.S. Food and Drug Administration (FDA) approved the drug, Epidiolex, for the treatment of seizures associated with two rare forms of epilepsy, Dravet syndrome, and Lennox-Gastaut syndrome[1]. The active ingredient in Epidiolex, cannabidiol (CBD), is a chemical compound found in marijuana, but is not associated with the euphoria caused by tetrahydrocannabinol (THC)[2]. Epidiolex is the first FDA-approved drug that contains a purified drug substance derived from marijuana.
Epidiolex was tested in three randomized clinical trials involving 516 patients with either Lennox-Gastaut syndrome or Dravet syndrome[3]. The FDA approved Epidiolex under Priority Review granted for Dravet syndrome, and Orphan Drug designation was granted for both Dravet syndrome and Lennox-Gastaut syndrome indications[4]. Clinical and nonclinical studies were also conducted to assess the potential abuse of Epidiolex because CBD is currently a Schedule I substance under the Controlled Substances Act, due to it being a chemical component of marijuana. Based on these studies, the FDA will make a recommendation to the Drug Enforcement Agency (DEA) regarding scheduling, and the DEA will make the ultimate scheduling determination.[5]
The approval of Epidiolex is an important milestone in the development of marijuana-derived medications. FDA Commissioner Scott Gottlieb M.D. stated: “This approval serves as a reminder that advancing sound development programs that properly evaluate active ingredients contained in marijuana can lead to important medical therapies.” He offered additional words of encouragement, as well as a warning to those developing marijuana-derived medications: “We’ll continue to support rigorous scientific research on the potential medical uses of marijuana-derived products and work with product developers who are interested in bringing patients safe and effective, high quality products. But, at the same time, we are prepared to take action when we see the illegal marketing of CBD-containing products with serious, unproven medical claims. Marketing unapproved products, with uncertain dosages and formulations can keep patients from accessing appropriate, recognized therapies to treat serious and even fatal diseases.”
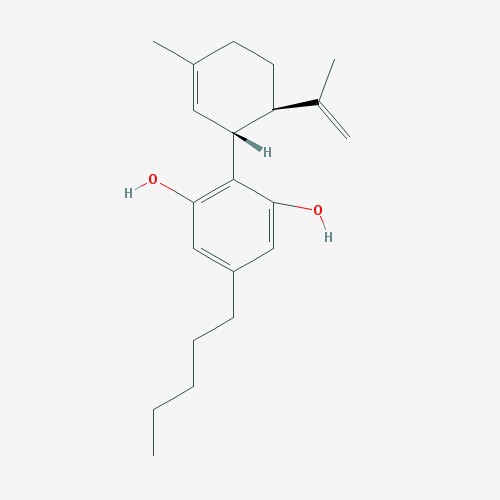
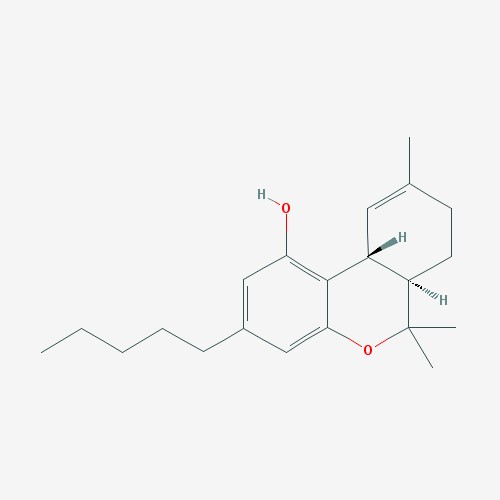
Cannabidiol is pictured above (left)[6]. THC is pictured above (right).[7]
[1] See https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm611046.htm
[2] Id.
[3] Id.
[4] Id.
[5] Id.
[6] See https://pubchem.ncbi.nlm.nih.gov/compound/cannabidiol#section=2D-Structure
[7] https://pubchem.ncbi.nlm.nih.gov/compound/16078#section=2D-Structure
